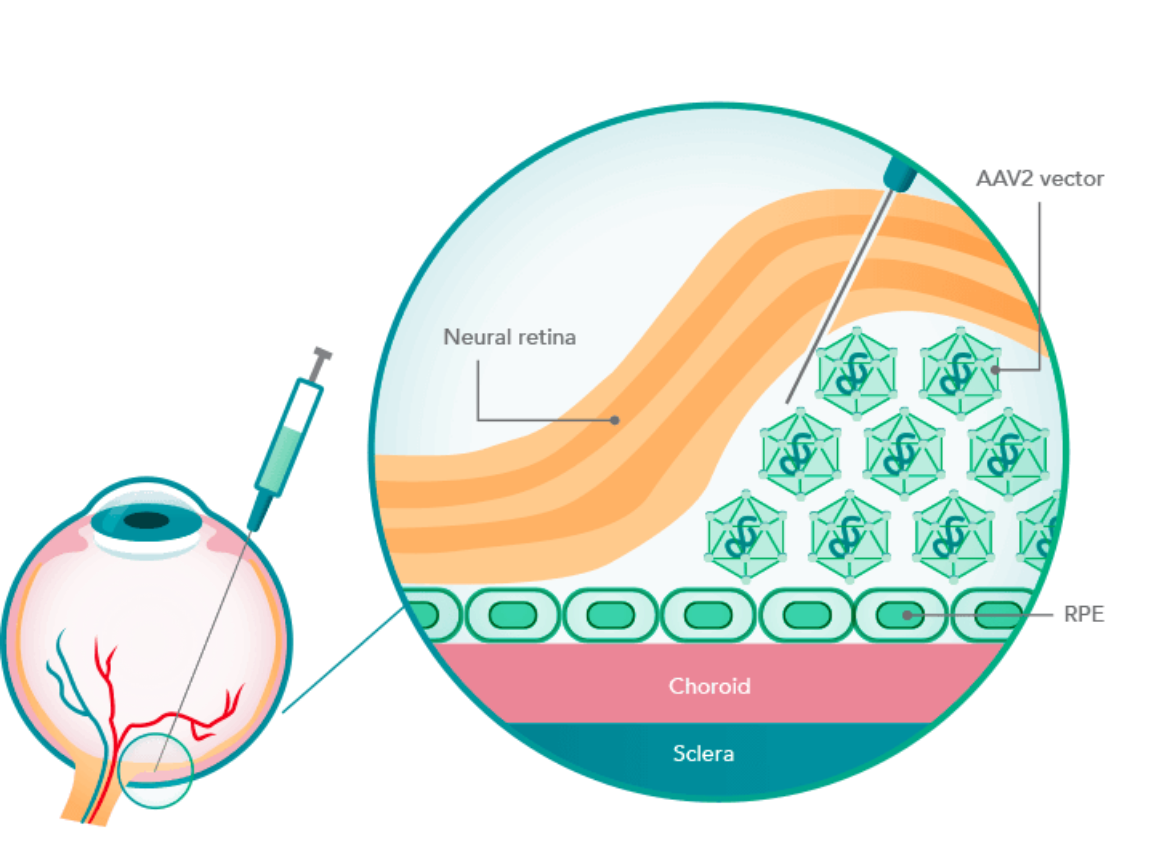
RPE65 gene delivery
RPE65 gene delivery
LUXTURNA uses the adeno-associated viral vector serotype 2 (AAV2) to carry a functional copy of the RPE65 gene into the retinal pigment epithelial (RPE) cells to compensate for the RPE65 mutation.2-4
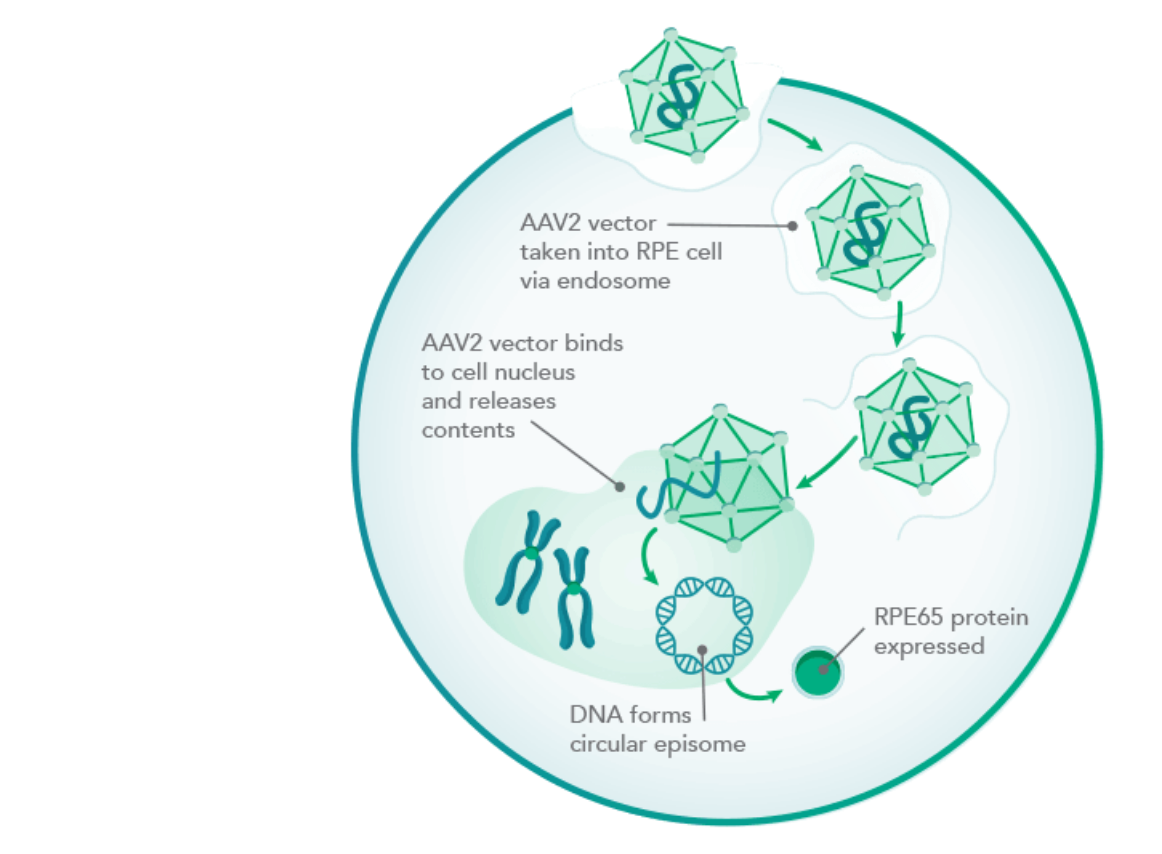
RPE65 protein production
RPE65 protein production
With a functioning RPE65 gene, the cells begin producing the RPE65 protein.2,5
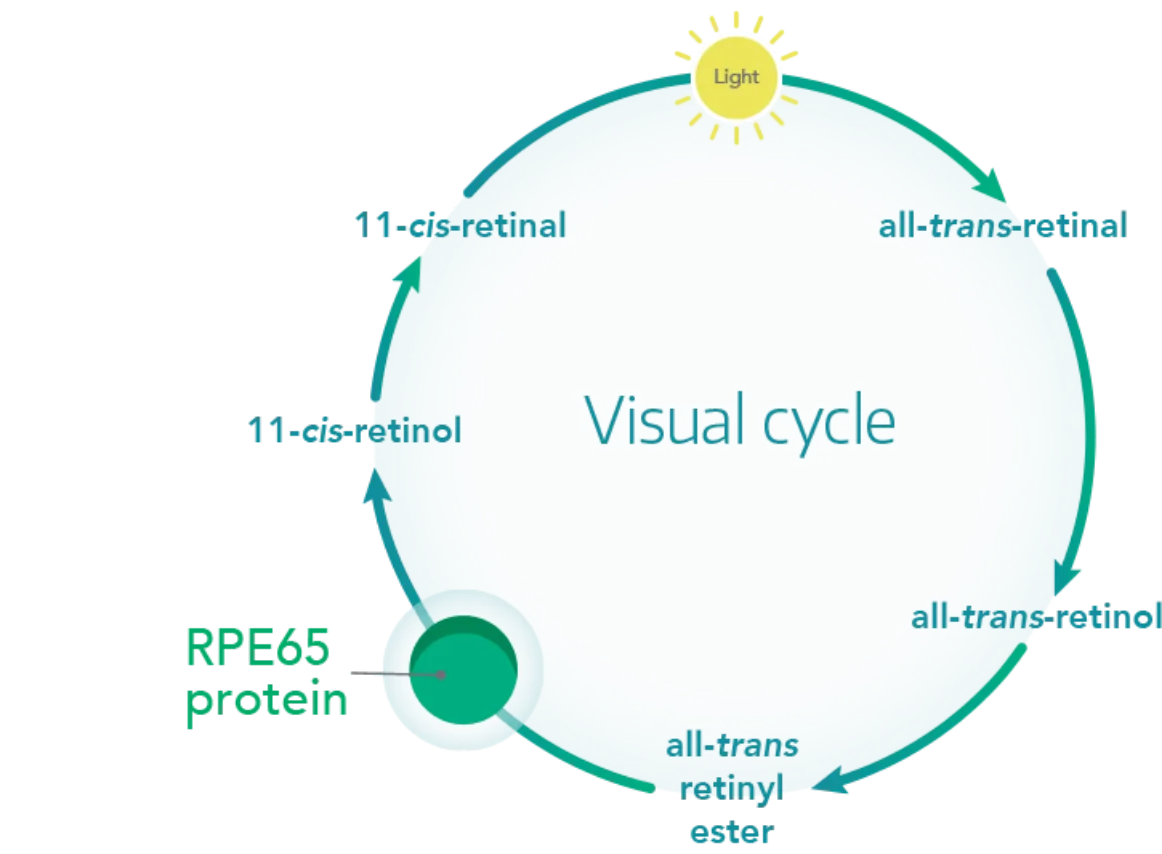
Restoring the visual cycle
Restoring the visual cycle
With the functional RPE65 protein, 11-cis-retinal (a critical visual pigment component) regenerates to restore the visual cycle.2,6