EFFICACYThe multi-luminance mobility testSM (MLMT®) results
One of the best things I have ever seen after surgery was the stars. I never knew that they were little dots that twinkled.
– Misty, study participant
For illustrative purposes based on actual patient experience. Results will vary.
LUXTURNA® improved functional vision based on a test that models activities of daily living1-3
Watch how LUXTURNA improved functional vision
At Year 1, participants treated with LUXTURNA demonstrated a statistically significant and clinically meaningful improvement in their ability to navigate at lower lux levels.1*
| Efficacy outcomes1 | LUXTURNA n=21 |
Control n=10 |
Difference (LUXTURNA – control) |
P value |
|---|---|---|---|---|
| MLMT® score change for bilateral eyes, median (min, max) |
2 (0, 4) | 0 (-1, 2) | 2 | 0.001 |
| MLMT score change for first-treated eye, median (min, max) |
2 (0, 4) | 0 (-1, 1) | 2 | 0.003 |
Using the MLMT to simulate everyday walking environments
The multi-luminance mobility test (MLMT) is a standardized, lab-based test in which participants were observed navigating a course with obstacles of varying height under different levels of illumination.1-3
BASELINE VISIT AT 1 LUX (FAIL)
1-YEAR VISIT AFTER LUXTURNA
ADMINISTRATION AT 1 LUX (PASS)
*The camera used automatically adjusts the level and temperature of light that it captures. Because of this feature, there may be slight variations in hue when filming at low light levels (eg, 1 lux). Both videos were filmed in low light environments. Note: This participant’s baseline passing level was 10 lux and 1-year passing level was 1 lux.
Lux levels were measured using a standardized and calibrated light meter.3
Demonstrated efficacy at year 1 with LUXTURNA1,2
55%of all participants had an MLMT score change of 2 or greater (16 out of 29)6*
of participants (8 of 9) who subsequently crossed over passed at 1 lux 1 year posttreatment6
of participants (13 of 20) in the intervention group successfully completed the MLMT at the lowest light level of 1 lux, while no participants in the control group passed at this level 1 year posttreatment2,6
*Intention-to-treat (ITT) population intervention (n=21) vs control (n=10).1
Improvements in functional vision have been sustained for 5 years—with observations ongoing.4,5 Follow-up will continue for up to 15 years postadministration.2,7
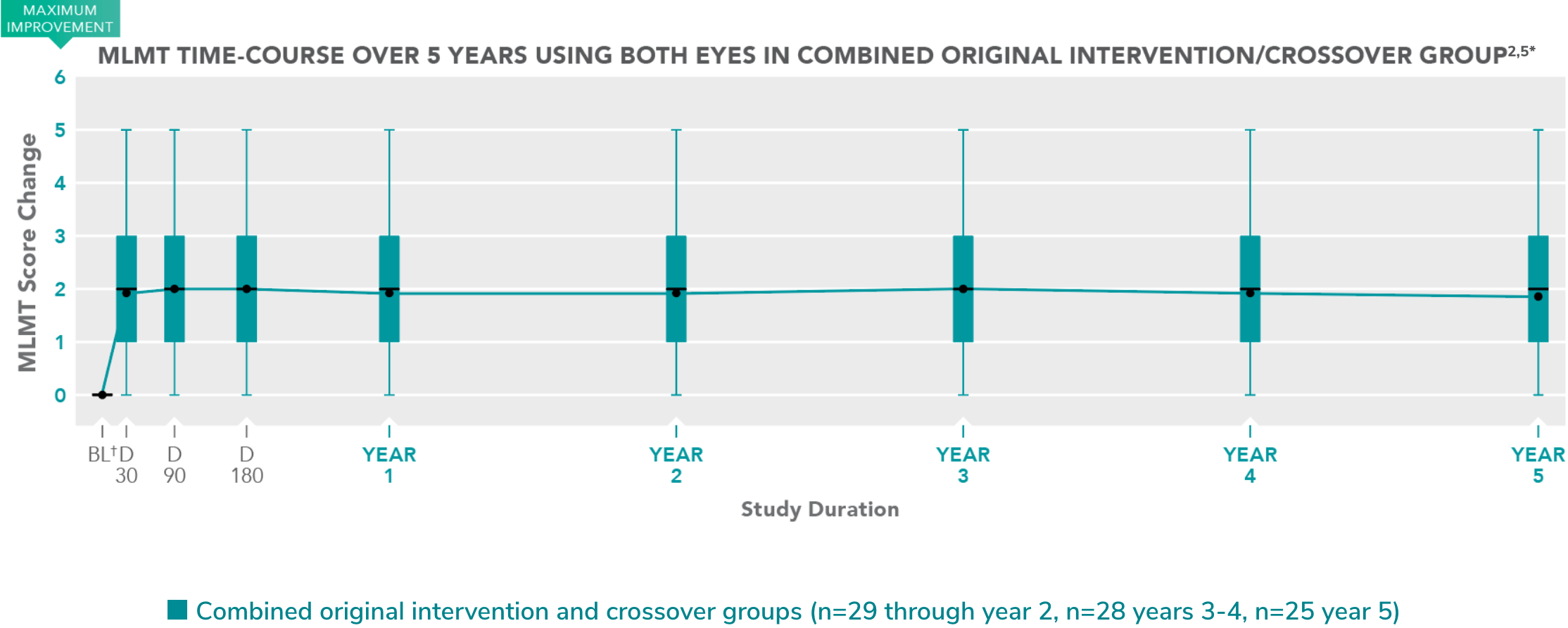
*Modified intention-to-treat (mITT) population.
†Baseline (BL) represents last MLMT measurement before treatment with LUXTURNA.
Each box represents the middle 50% of distribution of MLMT score change. Vertical lines represent additional 25% above and below the box.
The horizontal bar within each box represents the median. The dot within each box represents the mean.
